Activation Energy Can Be Described as
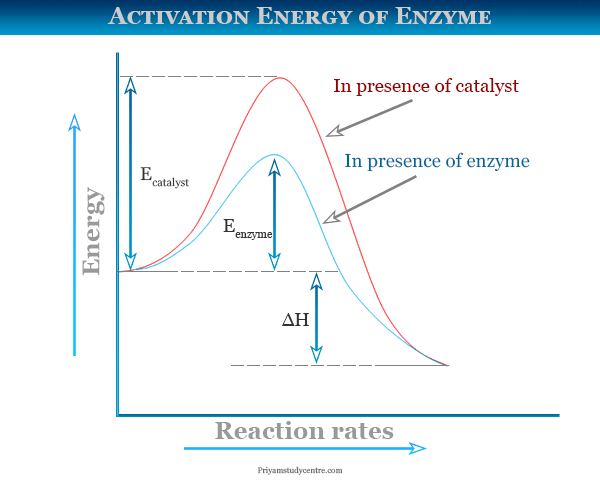
This energy is referred to as the activation energy E a which represents the minimal or threshold energy of the reaction. Reducing the activation energy can increase the rate of a reaction.
Activation Energy Definition Formula Diagram Examples
Activation energy can best be described as.

. See the answer See the answer done loading. The energy level of the products. For a reaction to occur the energy associated with the collision must equal or exceed the potential energy of the system at the top of the hill.
As such activation energy can be described as a potential barriers height that separates two minima of the reactants and the reaction products potential energy. The unstable high PE structural arrangement of atoms d. Activation energy in chemistry the minimum amount of energy that is required to activate atoms or molecules to a condition in which they can undergo chemical transformation or physical transport.
Activation energy can be described as _____. It can be described as the potential energy hill or barrier. In transition-state theory the activation energy is the difference in energy content between atoms or molecules in an activated or transition-state configuration and the.
Show Answer Hide Answer DIRECTIONS. Activation energy can best be described as. Activtin energy cn best be described s.
Activation Energy originated in physics and chemistry describing the minimal amount of energy required for a reaction to occur. But before the reactants can be converted into products the free energy of the system must overcome the activation energy for the reaction as shown in the figure below. O There is no relationship between activation energy and rate of a reaction.
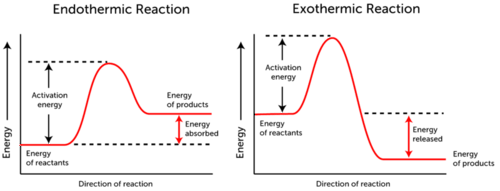
Group of answer choices a The amount of energy lost in an exothermic reaction. Activation energy can be described as _____. The minimum PE difference between the activated complex and the reactants.
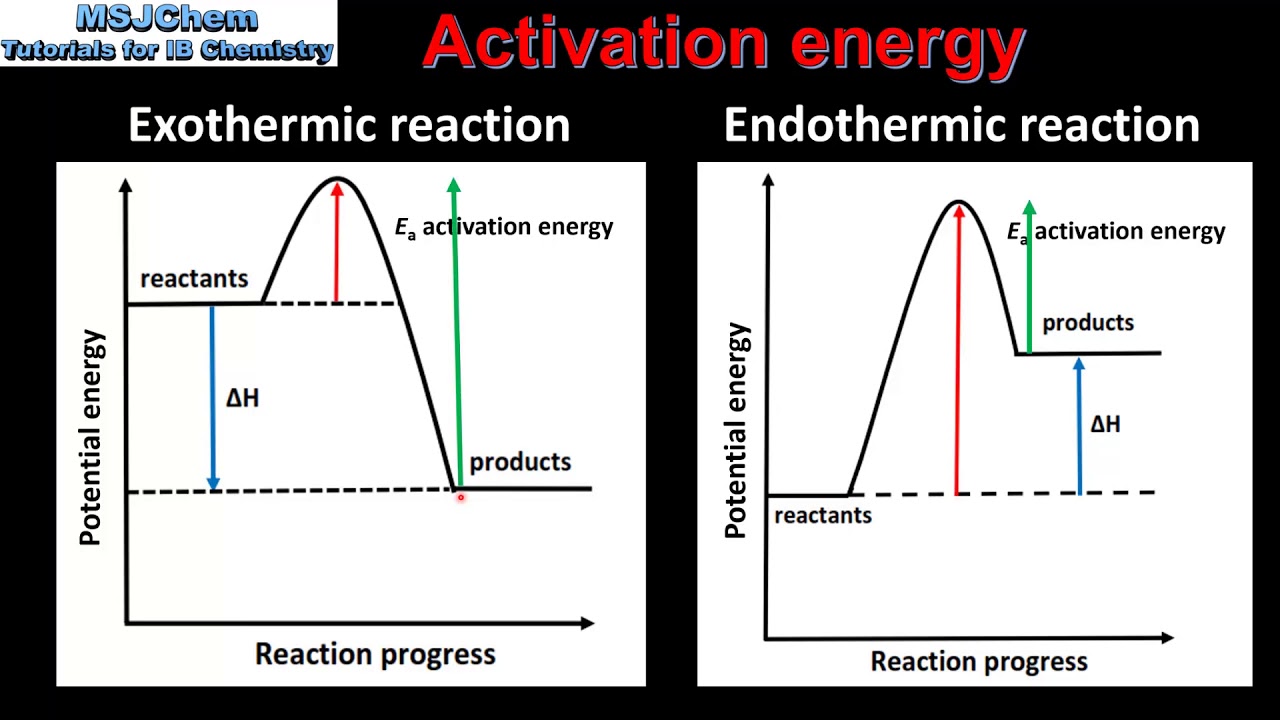
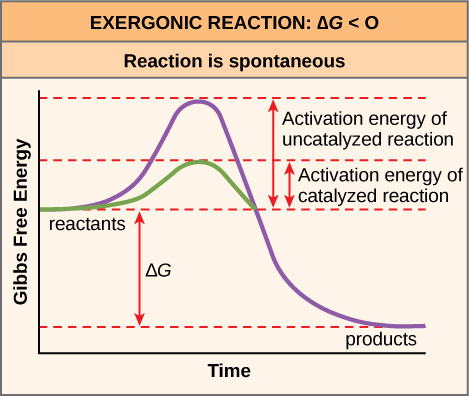
O Reducing the activation energy can decrease the rate of a reaction. A numerical description of the amount of energy needed by colliding reactant molecules in order to form products. The activation energy E a labeled Δ G in Figure 2 is the energy difference between the reactants and the activated complex also known as transition state.
Posted by Anonymous March 22 2021 March 22 2021. Activation Energy kJ ΔH kJ A. Activation energy is the energy that a reaction Federal minimum wage law supersedes state minimum QUESTION 5 The graph below shows the energy changes duri VRAAG 8 Ammoniak is n kleurlose gas wat bekend is vir s.
The activation energy in the Arrhenius equation can best be described as asked Jun 26 2017 in Chemistry by maju88 a. Group of answer choices a The amount of energy lost in an exothermic reaction. A numerical description of the amount of energy needed by colliding reactant molecules in order to form.
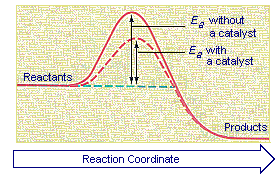
BYJUS Online learning Programs For K3 K10 K12 NEET. A substance that increases the rate of a reaction without appearing in the equation for the overall reaction is aan A. Question 10 1 point Activation energy can be described as the Energy difference between the reactants and the activated complex Energy provided by the catalyst Energy of motion Energy difference between the reactants and the products.
In a chemical reaction the transition state is defined as the highest-energy state of the system. The energy of the activated complex. Energy needed to activate the reactants and trigger a chemical reaction Which choice describes a laboratory technique that scientists might use to mimic a natural genetic process of cells.
The difference in energy between reactants and products. Activation energy can best be described as A the energy level of the reactants B Activation energy can best be described as a the School East Carolina University. Mtch ech vcbulary wrd t.
STARTING OUT IS EFFORTFUL. Save and Exit Save and extA Submit Mark this and return 1 See answer. Activation Energy can be viewed as the disproportionately high energy required to get something moving compared to keeping it moving.
Activation energy can be. For the reaction to proceed at a certain rate there must be a sufficient number of molecules the energy of which is equal to or higher than the activation energy. The energy level of the reactants.
The Activation Energy can best be described as. The activation energy is the difference between the energy of the reactants and the maximum energy ie. The maximum energy level of the reaction.
The difference in energy between reactants and the maximum energy. The energy of the activated complex b. The Activation Energy can best be described as.
A numerical description of the amount of energy released by colliding reactant molecules when they form. The vertical axis in this diagram represents the free energy of a pair of molecules as a chlorine atom is transferred from one to the other. A energy needed to activate an enzyme B potential energy C energy released by a chemical reaction D metabolism E energy needed to activate the reactants and trigger a chemical reaction.
Activation energy is described as a. For broader application you can visualise it as the initial hill or expenditure. The reaction between textH_2 textg and textF_2 textg Figure 124 needs energy in order to proceed and this is the activation energy.
A point on the PE diagram where KE PE c. B The amount of energy gained in an endothermic reaction c All of the above d The amount of energy required to start a reaction.
Activation Energy Ck 12 Foundation

6 2 2 Define The Term Activation Energy E A Youtube

Activation Energy Article Khan Academy

Lesson Explainer Enzyme Action Nagwa

18 4 Potential Energy Diagrams Chemistry Libretexts

Activation Energy Article Khan Academy
What Is Difference Between Endothermic And Exothermic Reaction If Both Require Activation Energy Quora
6 1 Activation Energy Sl Youtube

Activation Energy And Temperature Dependence Boundless Chemistry

Difference Between Free Energy And Activation Energy Compare The Difference Between Similar Terms

Activation Energy Definition Facts Britannica







Comments
Post a Comment