Under Which of the Following Conditions Will Vaporization Best Occur
Heat of vaporization b. 8 Under which of the following conditions will vaporization best occur.

The Outer Planets Dust And Plasma Outer Planets Earth And Space Science Physics
Direct conversion from solid to vapour is called sublimation.

. Under which of the following conditions will vaporization best occur. None of the above 6. Weak forces between molecules high kinetic energy large surface area small surface area low kinetic energy low molecular mass high mass large surface area high kinetic energy high molecular energy.
The equilibrium pressure exerted by the vapour in a closed container is called the vapour pressure. Which probably has the highest boiling point at 100 atm pressure. Normal boiling point c.
When a liquid is in dynamic equilibrium with its vapor at a given temperature the following conditions could exist. Under which of the following conditions will vaporization best occur. B Small surface area low kinetic energy low.
Transcribed Image Textfrom this Question. If the reaction is run in a cylinder with a movable piston decreasing the volume will cause Which one of the following. Vaporization conversion of a substance from the liquid or solid phase into the gaseous vapour phase.
Weak forces between molecules high kinetic energy large surface area small surface area low kinetic energy low molecular mass high mass large surface area high kinetic energy high molecular. With the increase in surface area the rate of vaporization also increases as more number of particles is exposed to the change in temperature. The vapour pressure decreases as the temperature decreases but theoretically at least there will always be some molecules with enough energy to escape into the vapour state.
The vapor pressure of a liquid e. Evaporation is a phase transition from the liquid phase to vapour a state of substance below critical temperature that occurs at temperatures below the boiling temperature at a given pressure. II The vapor pressure has a unique value.
Up to 256 cash back 6 Nov 2019. Which probably has the highest boiling point at 100 atm pressure. High mass large surface area and high kinetic energy b.
Weak forces between molecules high kinetic energy large surface area small surface area low kinetic energy low molecular mass high mass large surface area high kinetic energy high molecular energy small surface area low kinetic energy strong molecular forces large. Which one of the following DECREASES as the strength of the attractive intermolecular forces INCREASES. III The opposing processes liquid to vapor and vapor to liquid proceed at equal rates.
Low kinetic energy strong intermolecular forces and large surface area e. Under which of the following conditions will vaporization best occur. Pressure is inversely propositional to evaporation.
A Weak forces between molecules high kinetic energy large surface area. Weak intermolecular forces high kinetic energy and large surface area c. Heat must be supplied to a solid or liquid to effect vaporization.
A small surface area low kinetic energy low molecular mass b low kinetic energy strong molecular forces large surface area c weak forces between molecules high kinetic energy large surface area d high molecular energy small surface area e high mass large surface area high kinetic energy. Evaporation only occurs when the partial pressure of vapor of a substance is less than the equilibrium vapor pressure. For example due to.
If conditions allow the formation of vapour bubbles within a liquid the vaporization process is called boiling. I There is no transfer of molecules between liquid and vapor. Which one of the following salts gives a basic aqueous solutions.
High molecular energy and small surface area d. As pressure increases it gets difficult for the particles to gain the required kinetic energy and escape. The liquid is evaporating.
Under which of the following conditions will vaporization best occur. Under which of the following conditions will vaporization best occur. The sublimation temperature of a solid d.
Under which of the following conditions will vaporization best occur. Evaporation occurs on the surface. Up to 256 cash back Under which of the following conditions will vaporization best occur.

Strange Matter Chemistry Education Chemistry Classroom Physics And Mathematics

States Of Matter And Phase Changes Doodle Notes Distance Learning States Of Matter Doodle Notes Distance Learning
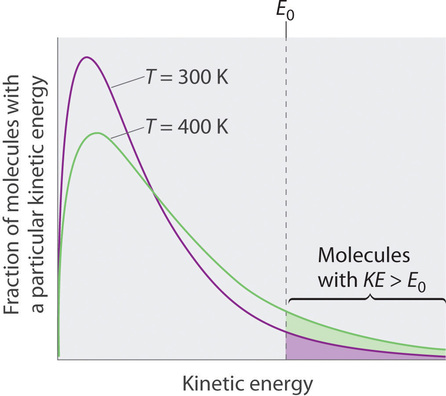
11 5 Vaporization And Vapor Pressure Chemistry Libretexts

Solved Which Probably Has The Highest Boiling Point At 1 00 Chegg Com

How To Calculate Molar Mass Molecular Weight Youtube Molar Mass Molecular Mass Molecular
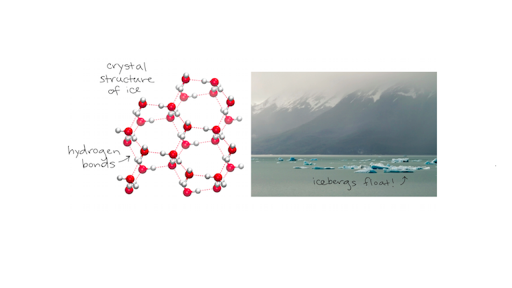
Specific Heat Heat Of Vaporization And Density Of Water Article Khan Academy

States Of Matter And Phase Changes Doodle Notes Doodle Notes States Of Matter Graphic Organizers

Pin By Tipsycat On 1 Home Remedies For Gas Gas Remedies Gas Relief

11 5 Vapor Pressure Chemistry Libretexts

What Can You Do To Reduce The Risk Of Having Kidney Stones St Pete Urology Kidney Stones Calcium Rich Foods Kidney

States Of Matter Vocabulary Matching Worksheet Matter Vocabulary Science Lessons Next Generation Science Standards

Intermolecular Forces And Vapor Pressure Video Khan Academy

Vapor Pressure Video States Of Matter Khan Academy

What Do You Do For A Kidney Stone St Pete Urology Kidney Stones Kidney Kidney Health

Phase Change Infographic States Of Matter Chemical And Physical Changes Learn Physics

Double Tempered Glass Door Convenient And Durable Plastic Handle Detachable Door Seal Hidden Magnetic Stripe Inside The Door Double Layer Tempered G ท กอย าง

Ngss 5 Ps1 1 States Of Matter Ngss Aligned Lesson Modeling Matter States Of Matter Matter Science Ngss



Comments
Post a Comment